Nanotechnology
is the science of the small, the very small. No longer do we have to depend on nature for raw materials. We can now create our own materials and fashion nature in a way that suits our needs. The promise of nanotechnology is boundless. Our research team while looking for ways to create nanobots, nanoscale man-made machines that can enter the bloodstream and carry out critical procedures such as delivering drug treatments or repairing damaged tissue, discovered the bacteriophage. A natural occurring organism, a nanobot, that targets infection and eliminates it within hours. This organism could then be genetically modified to make it both patentable and effective in targeting specific infectious diseases.
The world is in crisis, deaths from infectious diseases are a leading cause of death worldwide. The solution is a genetically modified organism designed to seek out the bacteria cells that cause infections and destroy them. Genetically modified bacteriophage, genetically modified to replicate faster, results in the faster destruction of staph cells, leading to faster recovery time for patients. The bacteriophage lands on a staph bacteria cell and injects its DNA into the inner workings of the cell. The DNA then replicates into thousands of new bacteriophages that spread rapidly to other infection cells and grow exponentially, destroying all signs of infection.
There are few barriers in the manufacturing process of bacteriophages, as they are fast to market with a long shelf life. This makes bacteriophages the next-generation treatment for antibiotic-resistant infectious diseases. Besides developing a bacteriophage for Staphylococcus, Viridyne Genetics has also developed bacteriophages for Streptococcus and Tuberculosis as well. Viridyne Genetics is now firmly poised to become the global leader in the antibiotic-resistant infectious disease treatment market. “Viridyne Genetics, the future of medicine.”
GENETICALLY MODIFIED BACTERIOPHAGE (BIO-PHAGE)
Bacteriophage VST has been identified to target Staphylococcus aureus (MSSA & MRSA) bacteria. A genetically modified and enhanced VST is the invention hereafter described. The DNA of the VST has been altered by splicing in a gene sequence that increases the Bacterio-phage’s ability to replicate faster, thereby increasing its numbers and increasing its effectiveness in eliminating Staphylococcus bacteria cells. The invention is a unique Genetically modified organism that can be programmed to target various harmful life threatening infectious bacteria.
Global death rates from antibiotic-resistant infections continue to increase.
WE HAVE A SOLUTION!!

Bacteriophage are viruses that parasitize and kill specific bacteria (Bacteriophage VST on the right).
BACTERIOPHAGE FOR THE TREATMENT OF INFECTIONS INCITED BY STAPHYLOCOCCUS AUREUS
METHICILLIN-SENSITIVE AND METHICILLIN-RESISTANT FORMS OF STAPHYLOCOCCUS AUREUS (MSSA & MRSA)
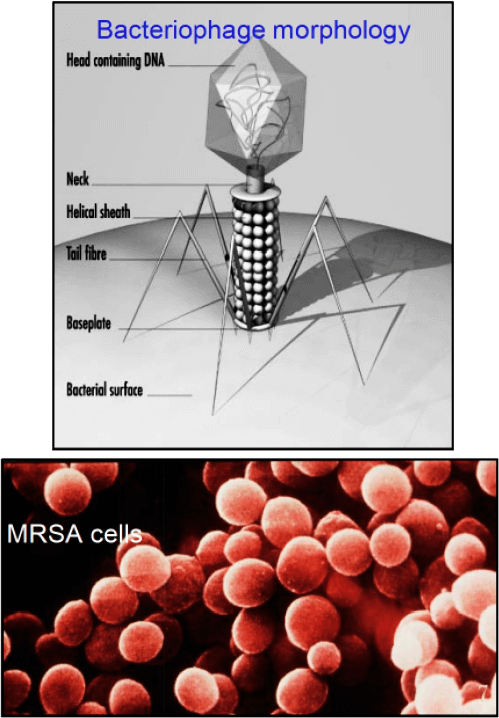
Viruses that parasitize and kill only specific bacteria.
Considered as ‘Biopharmaceuticals’ by the US FDA.
Non-parasitic to other microbes, or to plants or animals.
Non-toxic and non-immunogenic to humans.
Approved by US FDA and EPA for food and environmental uses.
Bacterial resistance to bacteriophage is minimal and expected.
Manufacturing costs are lower than for antibiotics.
Represent a new class of antimicrobials.
There are no new safe and effective antibiotics on the horizon.
All efforts to develop a useful Staph vaccine have failed.
BIOPHARMACEUTICAL PRODUCTS
BACTERIOPHAGE PRODUCTS FOR THE TREATMENT OF:
RESPIRATORY INFECTIONS
(S. AUREUS-INCITED PNEUMONIA: MRSA¹ & MSSA²-VAP³
SYSTEMIC INFECTIONS
(S. AUREUS-INCITED BACTEREMIA: MRSA & MSSA BACTEREMIA
¹MRSA = Methicillin-Resistant Staphylococcus aureus
²MRSA = Methicillin-Sensitive Staphylococcus aureus
³VAP = Ventilator-Associated Pneumonia
BACTERIOPHAGE PRODUCT FOR THE TREATMENT OF MRSA/MSSA -VAP✲
✲(Ventilator-Associated Pneumonia)
STATEMENT OF INTENDED USE (FOR US FOOD AND DRUG ADMINISTRATION):
A bacteriophage-based biopharmaceutical product for the treatment of Ventilator-Associated Pneumonia (VAP) as incited by Staphylococcus aureus
BACTERIOPHAGE THERAPEUTIC PRODUCT FOR THE TREATMENT OF:
Community-Associated MRSA (CA-MRSA)-Incited VAP
Hospital-Acquired MRSA (HA-MRSA)-Incited VAP
Methicillin-Sensitive Staphylococcus aureus (MSSA)-Incited VAP
VENTILATOR-ASSOCIATED PNEUMONIA (VAP):
VAP is pneumonia in a person assisted by a ventilator within the past 48 hours.
VAP is a life-threatening infection.
Individuals with VAP are critically ill.
The death rate is very high.



AN OVERVIEW OF VENTILATOR-ASSOCIATED PNEUMONIA (VAP)
VAP ACCOUNTS FOR ABOUT:
15% of all nosocomial (hospital-acquired) infections.
27% of all infections in the coronary care and intensive-care units.
86% of all cases of nosocomial pneumonia.
NOTE:
The primary risk factor for hospital-acquired bacterial pneumonia is mechanical ventilation.
The risk for pneumonia increases 3- to 10-fold in patients on mechanical ventilation.
VAP is especially common in people with ARDS or COPD.
VAP prolongs ICU stay and increases the risk of death in critically ill patients.
The mortality rate for VAP is as high as 43% with resistant microbes.
The added cost of VAP in the US is about $40,000 per patient, or about $1.2 billion per year.
VAP IS:
The leading cause of death among all nosocomial infections.
Responsible for increased morbidity and mortality, hospital time and hospital costs.
INFECTIOUS AGENTS:
Common infectious bacteria for which the Company is developing novel therapeutic products include S. aureus and other Staphylococcal (Staph) species, many strains of which are now resistant to most commercially-available antibiotics. The health threat and economic consequences of many common infections are now catastrophic in both the developing and the developed world.
STAPHYLOCOCCUS AUREUS:
S. aureus is a common bacterium that may be found on the skin and in the nasal passages of healthy people. It is known to incite serious, life-threatening infections of the respiratory tract and cardiovascular system, including the blood stream, plus infections of bone, soft tissue, skin and eye. Forms of S. aureus that are resistant to methicillin and other antibiotics are known as Methicillin-Resistant Staphylococcus aureus (MRSA). Vancomycin is generally used to treat resistant Staphylococcal infections with some success. However, a more resistant form of S. aureus, known as Vancomycin-Intermediate-Resistant S. aureus (VISA) has emerged to complicate treatment. The most troublesome form of antibiotic-resistant S. aureus is known as Vancomycin-Resistant S. aureus (VRSA).
METHICILLIN-RESISTANT STAPHYLOCOCCUS AUREUS (MRSA):
MRSA is an antibioticresistant form of S. aureus that is responsible for infections in patients of all ages. Infection by MRSA is typically associated with particular settings, such as hospitals and long-term care facilities, and is common among patient groups that have prolonged hospitalizations, past antimicrobial use, indwelling catheters, decubitis ulcers and postoperative surgical wounds, as well as those who require intravenous drugs or treatment with enteral feedings or dialysis. Infections incited by MRSA present a considerable dilemma to clinicians, since therapeutic options are limited and suboptimal dosing contributes to greater resistance, heightened mortality and increased length of hospital stay. For decades, glycopeptide antibiotics, such as vancomycin, were considered to be the only agents to which MRSA had not developed resistance. However, as a result of the overuse of glycopeptide antibiotics, MRSA forms have now emerged with reduced or limited susceptibility to these agents. Revised clinical definitions and new molecular approaches allow investigators to distinguish Community-Associated MRSA (CA-MRSA) infections from traditional Nosocomial (hospital-acquired) MRSA (HA-MRSA). Therapeutic options for these infections are limited, so the potential exists for continued high morbidity and mortality. Transmission occurs by contact, such as in day-care centers and on Indian reservations, as well as among athletes, military personnel and correctional inmates. These patients are otherwise healthy individuals, with no known risk factors for MRSA infection. Although infections resulting from the usual strains of bacteria continue to have high rates of cure, antibiotic-resistant strains have emerged in most countries, with case fatality rates of 40 to 60 percent. In this post-antibiotic era, alternative therapies are eagerly sought by governments, public health agencies and the health care industry.
METHICILLIN-SENSITIVE STAPHYLOCOCCUS AUREUS (MSSA):
MSSA is a form of S. aureus that is or may be resistant to many common antibiotics, but that is sensitive to methicillin. MSSA is also responsible for serious infections in patients of all ages. Infection by MSSA may be encountered in hospital, clinic or community settings, and is increasingly common among patient groups that have had recent hospitalizations or otherwise crowded conditions, such as among sporting teams, military situations, confinement or incarceration, or who were in close contact with animals. MSSA cannot be excluded from serious consideration when a Staph infection is being evaluated.
VENTILATOR-ASSOCIATE PNEUMONIA (VAP):
VAP is characterized as pneumonia of infectious origin in a patient on ventilator, which results in fluid accumulation in lung alveoli. VAP is distinguished from other pneumonias by the inciting pathogen, the antibiotic treatments administered, and the methods of diagnosis, prognosis and prevention. In order to have VAP, the patient must be on a ventilator. It is the fact that the patient is on a ventilator that defines VAP, not the infectious agent.
MRSA-INCITED VENTILATOR-ASSOCIATE PNEUMONIA (MRSA-VAP):
VAP that is incited by MRSA, one of the key pathogens associated with VAP, is referred to as MRSA-VAP. Patients on ventilator are already sick and highly likely to become infected with MRSA and develop MRSA-VAP, which has a high rate of mortality among all patients on ventilator, and most especially among the elderly.
MRSA-INCITED BACTEREMIA (MRSA-BACTEREMIA):
Bacteremia is the presence of bacteria in the blood, which may be caused by dental work, catheterization of the urinary tract, surgical treatment of an abscess or infected wound, or colonization of indwelling devices, especially intravenous and intracardiac catheters, urethral catheters or ostomy devices and tubes. Bacteremia secondary to infection usually originates in the genitourinary (GU) or gastrointestinal (GI) tract, or on the skin. Chronically ill patients, immunocompromised patients and injection drug users have an increased risk of bacteremia. MRSA is a common, dangerous and difficult-to-treat inciting agent of bacteremia.
MARKET OPPORTUNITY ANTI-INFECTIVES & BIOPHARMACEUTICALS
The anti-infectives market is a major therapeutic category, having global sales of about $45 billion, with the majority ($31 billion) in antibiotics.
The evolution of drug resistance is the most powerful driver of new anti-infectives.
The antibacterial market is the largest infectious disease sector.
Biopharmaceuticals are the new breed of medicines.
Over 130 biopharmaceuticals are in the market, including 13 blockbusters.
More than one third of all pipe-line products in development are biopharmaceuticals.
The 2009 biopharmaceutical market reached about $80 billion, up from previous years.
Biopharmaceutical markets could reach $100 billion by the end of the decade.
Biopharmaceuticals are expected to represent 17% of all prescriptions in 2010.
MRSA has developed some degree of resistance to all conventional antibiotics.
BACTERIOPHAGE DEFINITION:
Bacteriophage (phage) are viruses that infect and parasitize bacteria. Phage reproduce by inserting their DNA into the DNA of a host bacterial cell. The phage DNA directs the production of progeny phage by the host bacterium. The new phage burst from the host cell, killing it, and then infecting more bacteria. There are many types of phage, many of which are capable of eradicating its bacterial host. Phage are abundant in the biosphere. Phage only attack bacteria, and have not been found to have adverse effects on humans or other animals.
BACTERIOPHAGE TYPES:
There are two types of phage: lytic or temperate. Only lytic phage are useful for developing therapeutic phage preparations. Lytic phage multiply inside the bacterial cell and release new phage, lysing and killing the host bacterial cell in the process. In contrast, temperate phage integrate their DNA into the host bacterial DNA creating a prophage, which can escape and integrate into the DNA of another bacterial cell. Such phage are not appropriate for use in therapy because they can transfer virulence and antibiotic resistance genes from one bacterium to another.
BACTERIOPHAGE SAFETY:
Phage are considered as being safe for therapeutic use, with few if any side-effects having ever been reported. Pain was reported in one study, and was related to the rapid release of endotoxins as the phage lysed the bacteria, which is what happens also with antibiotic therapy.
BACTERIOPHAGE HISTORY:
Bacteriophage were discovered over 90 years ago by French and English scientists. It was quickly realized that phage had the potential to kill bacteria that cause many infectious diseases in humans, as well as in plants and animals. An institute for the study and production of phage was founded in the mid-1930s in the Soviet Republic of Georgia and remains active today. In the West, research in phage therapy has been limited, with nominal commercialization. Its use all but ceased in the 1940s, with the emergence of penicillin and other antibiotics.
In recent years there has been renewed interest in phage therapy, primarily because of the growing resistance of many strains of bacteria to existing antibiotics. The international literature contains several hundred reports on phage therapy, with the majority of the publications coming from researchers in the former Soviet Union and Eastern European countries.
The best studies are from the Institute of Immunology and Experimental Medicine of the Polish Academy of Sciences(Slopek S., et al., 1985₁, Slopek S., et al., 1987₂). Phage therapy was tested in 550 patients at 10 clinics in three cities. Major infecting agents included species of Staphylococcus, Pseudomonas, Escherichia, Klebsiella and Salmonella. Rates of success ranged from 75 to 100%. The success rate was about 94% for the 518 cases where antibiotic therapy was shown to be ineffective.
Medline citations published during 1966-1996 on the use of therapeutic phage in humans were reviewed in the Journal of Infection (Alisky J., et al., 1998₃). The overall success rate for phage therapy was reported to be in the range of 80-95%. Historically, bacteriophage were seldom subjected to scientifically valid evaluations.
MARKET OPPORTUNITY INFECTIOUS DISEASE FACTS US AND WORLDWIDE
INFECTIOUS DISEASES:
Leading cause of death worldwide (20 million/yr; ⅓ of total.
Third leading cause of death in the US.
Increase of more than 50% in the US since 1980.
Antibiotics are the most widely prescribed drugs in the US.
$130 billion/yr spent in the US for treatment of infectious diseases.
Antibiotic-resistant infections cost more than $10 billion/yr in the US.
More than 90% of Staph infections are antibiotic-resistant.
STAPHYLOCOCCUS AUREUS BACTERIOPHAGE (STAPH PHAGE) PRODUCTS FOR THE TREATMENT OF:
Respiratory Infections: MRSA-incited Ventilator-Associated Pneumonia (MRSA-VAP)
Systemic Infections: MRSA-incited Bacteremia (MRSA-Bacteremia)
MRSA-INCITED VENTILATOR-ASSOCIATE PNEUMONIA (MRSA-VAP):
VAP is characterized as pneumonia of infectious origin in a patient on ventilator, which results in fluid accumulation in lung alveoli. VAP is distinguished from other pneumonias by the inciting pathogen, the antibiotic treatments administered, and the methods of diagnosis, prognosis and prevention. In order to have VAP, the patient must be on a ventilator. It is the fact that the patient is on a ventilator that defines VAP, not the infectious agent. VAP that is incited by MRSA is referred to as MRSA-VAP. Patients on ventilator are already sick and highly likely to become infected with MRSA and develop MRSA-VAP, which has a high rate of mortality among all patients on ventilator, and most especially among the elderly.
MRSA-INCITED BACTEREMIA (MRSA-BACTEREMIA):
Bacteremia is the presence of bacteria in the blood, which may be caused by dental work, catheterization of the urinary tract, surgical treatment of an abscess or infected wound, or colonization of indwelling devices, especially intravenous and intracardiac catheters, urethral catheters or ostomy devices and tubes. Bacteremia secondary to infection usually originates in the genitourinary (GU) or gastrointestinal (GI) tract, or on the skin. Chronically ill patients, immunocompromised patients and injection drug users have an increased risk of bacteremia. MRSA is a common, dangerous and difficult-to-treat inciting agent of bacteremia.
THE PATHOGEN, STAPHYLOCOCCUS AUREUS
S. aureus and many other species of Staphylococcus (Staph) are common bacteria typically encountered on the skin and in the nasal passages of healthy people. Staph can cause serious, life-threatening infections of the respiratory tract and cardiovascular system, including the blood stream.
- Slopek S, Kucharewicz-Krukowska A, Weber-Dabrowska B, Dabrowski M. 1985. Results of bacteriophage treatment of suppurative bacterial infections. IV. Evaluation of the results obtained in 370 cases. Arch Immunol Ther Exp (Warsz). 33(2):219-40.
- Slopek S,Weber-Dabrowska B, Dabrowski M, Kucharewicz-Krukowska A. 1987. Results of bacteriophage treatment of suppurative bacterial infections in the years 1981-1986. Arch Immunol Ther Exp (Warsz). 35(5):569-83.
- Alisky, J., Iczkowski, K., Rapoport, A., Troitsky,N. 1998. Bacteriophages Show Promise as Antimicrobial Agents. J of Infection 36(1):5-15.
WORLD INFECTIOUS DISEASE CRISIS (WHO, NIH, CDC)
THE PRIMARY THREATS TO WORLD PUBLIC HEALTH
Increased resistance to antibiotics by MRSA.
Spread of Tuberculosis (MDRTB/XDRTB), HIV/AIDS & Malaria.
Spread of Antibiotic-Resistant Pseudomonas aeruginosa.
OF 55 MILLION DEATHS IN THE WORLD EACH YEAR:
20 million — infectious diseases
15 million — circulatory diseases
6 million — cancer
3 million — other respiratory diseases
11 million — various other causes
ESTIMATED MARKET OPPORTUNITY
The international infectious disease therapeutics market grew from about $16 billion in 1991 to more than $45 billion in 2007, and continues to grow. The USmarket share has grown from about 31% to about 37%, while the Japanese market share has declined from about 26% to about 21%. The European market share and the markets in the rest of the world have remained nearly stable during that period, with Europe represented by about a 28% market share, and the rest of the world about 15%. The US market share for newgeneration antibiotics alone is anticipated to exceed $15 billion within the next year. This phenomenal growth continues in spite of the fact that bacteria have now developed resistance to nearly all of the antibiotic agents that represent the product growth leaders. This has led to a continual stream of new antibiotic products introduced to the market, many of which have a greatly shortened product life as a direct result of the rapid development of resistance.
The medical community, including national and international public health agencies, has been urging the biomedical research community to expand their efforts to identify new technologies and products employing novel mechanisms of action against infectious bacteria. The underlying technology surrounding products is anticipated to yield multiple new therapeutic agents for the treatment of sensitive and resistant forms of various bacterial diseases. The development and marketing of new antibacterial products that have novel mechanisms of action are less likely to elicit the development of resistance and will represent one of the most substantial market opportunities and perhaps some of the most medically useful products in modern human health care.
In US hospitals it is estimated that perhaps 3,000,000 patients are infected each year by bacterial pathogens, and from 80,000 to 100,000 people die from infections, compared with a yearly mortality of about 8,000 in the early 1990s by infectious diseases. About 90% of Staphylococcal infections, which are responsible for about 15% of all bacterial infections, are now resistant to penicillin, and more than 80% are resistant to methicillin.
ADVANTAGES OF BACTERIOPHAGE VS. CONVENTIONAL ANTIBIOTICS
Safety, Efficacy & Cost-Efficiency = Favorable Pharmacoeconomics
HIGH BACTERIAL TARGET SPECIFICITY:
Bacteriophage target specific bacteria; do not kill beneficial bacteria.
LOW TOXICITY:
Bacteriophage are non-toxic; may be administered in a few high doses.
LOW-COST PRODUCT DEVELOPMENT = HIGH PROFIT MARGIN
Bacteriophage products are less costly to Discover, Develop and Manufacture.
GREATER BIODISTRIBUTION:
Bacteriophage have greater tissue penetration in more tissue types for more effect, assuring greater bacterial kill, making subsequent infections with the same pathogen unlikely.
SELF-REPLICATION AT THE SITE OF INFECTION:
Bacteriophage replicate at the site of infection until the infectious agent is eliminated.
SELF-LIMITING:
Bacteriophage are eliminated by the immune system after the infection is cleared.
LONGER SHELF LIFE:
Bacteriophage are highly stable and have a long shelf life.
LONGER PRODUCT LIFE:
Bacterial resistance to bacteriophage develops at a low rate.
MULTIPLE PRODUCT FORMATS FOR A SINGLE BACTERIOPHAGE DRUG SUBSTANCE:
The same Bacteriophage Drug Substance may be used for multiple product formats (i.e., for Systemic, Inhalation, Topical or Ophthalmic administration).
STAPHYLOCOCCUS BACTERIOPHAGE PRODUCT SAFETY TESTING
INFECTIOUS DISEASES:
GENOTOXICITY TESTS: NO GENOTOXICITY
MUTAGENICITY TESTS:
Bacterial Mutagenicity Test
No mutagenicity detected
Mouse Lymphoma Assay
No mutagenicity detected
Micronucleus Assay
No mutagenicity detected
SAFETY PHARMACOLOGY: NO TOXICITY
SAFETY PHARMACOLOGY:
Neuropharmacological profile in rats
No biologically relevant changes
Cardiovascular (Hemodynamic) evaluation in dogs
No biologically relevant changes
Airway resistance and lung compliance in guinea pig
No biologically relevant changes
IMMUNOTOXICITY TESTS: NO IMMUNOTOXICITY
NATURAL KILLER (NK) CELL CYTOTOXICITY ASSAY:
No Cytotoxicity detected
Phage caused a dose dependent increase in NK activity compared to the control